The medical device industry isn’t just about machines and technology—it’s about improving lives. These devices are essential for diagnosing, treating, and managing various health conditions, from simple tools like bandages to complex surgical systems. With our population getting older and chronic illnesses becoming more common, the need for innovative medical devices is growing. They’re not just important for individual health, they’re also crucial for the economy.
The contract manufacturers are key players in this field, bringing ideas to life. The partnership between companies that design medical devices and those that specialize in making them lets designers focus on what they do best—coming up with new ideas and getting them out there. Manufacturers handle the nitty-gritty of making sure everything is up to scratch, from meeting regulations to managing the supply chain.
What makes a great contract manufacturing company? It’s all about pushing the boundaries of what’s possible, using the best materials and methods to create devices that work better. Reliability is key because these devices have to be perfect to keep patients safe. And of course, they have to meet all rules and regulations, with no exceptions. Plus, they need to offer a full package, from design to production, to support after the product is out there. The best companies not only meet these standards but exceed them, making sure every device they help create makes a real difference in healthcare.
What is Medical Device Contract Manufacturing?
Medical device contract manufacturing involves a company hiring another company to make their devices for them. The first company called the original equipment manufacturer (OEM), doesn’t have to worry about building the devices themselves. Instead, they rely on the expertise and resources of the contract manufacturer (CM). This setup helps the OEMs save time and money because the CMs are skilled at making these devices efficiently. Plus, CMs can handle everything from designing and testing the devices to making sure they meet all the rules and regulations.
The Benefits of Using Contract Manufacturers for Medical Devices
Working with a contract manufacturer (CM) has many advantages for medical device companies. Here’s why:
- Cost Efficiency: Contract medical device manufacturers have the necessary equipment and efficient processes, which means they can make medical devices more affordable. This helps the original equipment manufacturer (OEM) save money on production.
- Focus on What Matters: Outsourcing manufacturing allows OEMs to put their time and effort into the essential parts of their business, like research, development, and marketing. This focus on core competencies is crucial for being able to develop new ideas and expand in the market.
- Get to Market Faster: Contract manufacturers are focused on quickly turning ideas into actual products. They have the processes and expertise to make prototypes and manufacture products fast. This speed can be a big advantage, getting a product from the idea stage to the market quickly.
- Access to High Tech: Contract medical device manufacturers keep up with the latest and greatest technologies. When companies partner with them, they get access to cutting-edge technologies without having to spend a lot of money on them.
- Compliance: Making sure medical devices meet all the rules and regulations is complicated. Contract manufacturers who specialize in the medical field know these compliance issues inside out. This helps the OEMs reduce the risk of not following the regulations.
- Flexibility: Medical device contract manufacturers can quickly change how much they produce based on the demand for a product. This flexibility is hard for OEMs to achieve if they try to do everything in-house.
Key Services Provided by Medical Device Contract Manufacturers
Medical device contract manufacturers offer a wide range of services tailored to the needs of the medical device industry:
- Design and Development: Collaborating with clients to refine device designs, providing engineering expertise, assisting with material selection, and creating prototypes for testing functionality.
- Manufacturing and Production: Undertaking the entire manufacturing process, from fabricating individual components to assembling fully functional devices, using processes like machining, molding, electrical assembly, and packaging.
- Quality Assurance and Testing: Implementing rigorous quality control measures and conducting extensive testing to ensure compliance with specifications and regulatory requirements, ensuring the highest standards of quality and safety.
- Regulatory Compliance: Assisting with navigating complex regulatory requirements, including documentation, adherence to standards like ISO 13485, and liaising with regulatory authorities to obtain approval for commercialization.
- Packaging and Sterilization: Providing specialized packaging solutions to maintain product integrity and safety during storage and transportation, along with sterilization services to eliminate microbial contamination and maintain product sterility.
- Supply Chain Management: Overseeing logistics, inventory management, and procurement activities to optimize efficiency and ensure the timely delivery of medical devices.
By offering these critical services, medical device contract manufacturers play a crucial role in facilitating the development, production, and commercialization of innovative and safe medical devices, allowing original equipment manufacturers to bring their products to market efficiently.
Factors to Consider When Choosing a Medical Device Contract Manufacturer
Selecting the appropriate medical device contract manufacturer (CM) is a critical decision for original equipment manufacturers (OEMs) in the healthcare sector. It necessitates thoughtful consideration of various essential factors to guarantee that the collaboration aligns with the rigorous demands of medical device production and contributes to the successful introduction of high-quality products to the market. Here are the key considerations OEMs should assess when picking a medical device contract manufacturer:
- Quality and Regulatory Compliance: Opt for a manufacturer with robust quality management systems, as indicated by ISO 13485 certification, and a proven track record of meeting regulatory standards such as those set by the FDA.
- Expertise and Experience: Prioritize manufacturers with specific expertise and a solid history in your product category. This ensures they comprehend the distinctive requirements and challenges associated with your devices.
- Scalability and Flexibility: Seek a manufacturer capable of adjusting production volumes promptly without compromising quality. This capability ensures adaptability to changing market demands.
- Project Management and Communication: Effective project management and transparent communication are crucial. The chosen manufacturer should provide clear timelines, regular updates, and efficient risk management throughout the collaboration.
- Geographic Location: Evaluate the manufacturer’s location and its impact on logistics, shipping costs, and communication ease. Strike a balance between proximity benefits and considerations of quality and expertise.
Choosing the right medical device contract manufacturer involves a nuanced decision-making process, requiring careful assessment of quality, expertise, scalability, project management, and geographic location. By prioritizing these factors, OEMs can forge a productive partnership that guarantees their products meet the highest standards of quality and regulatory compliance, are efficiently delivered to the market, and ultimately contribute to improved patient care.
Top Medical Device Contract Manufacturing Companies
| Company | Location | Year Founded | No. of employees |
| Tegra Medical | Massachusetts, US | 2007 | 250-449 |
| Cadence Device | Virginia, US | 1985 | 500-999 |
| Coghlin | Massachusetts, US | 1885 | 500-999 |
| Providence Enterprise | Hong Kong. China | 1992 | 1000+ |
| Donatelle | Minnesota, US | 1967 | 250-499 |
| Janco Electronics Inc. | New Hampshire, US | 1959 | 100-249 |
| BMP Medical | Massachusetts, US | 1978 | 50-199 |
| BihlerMed | New Jersey, US | 2006 | 50-199 |
| HDA Technology | California, US | 1983 | 10-49 |
| DeviceLab | California, US | 2001 | 10-49 |
Tegra Medical
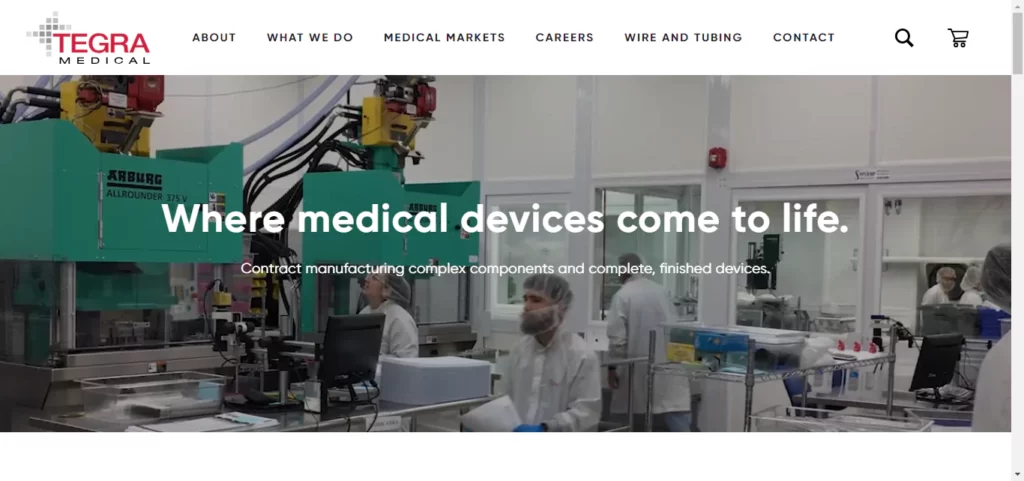
Tegra Medical is a well-known contract manufacturing solutions provider for the medical device industry. The company is often referred to as the place “where medical devices come to life.” Its clients depend on Tegra Medical for end-to-end manufacturing services that include everything from complex components to finished devices. Tegra Medical’s services cover all aspects of the manufacturing process, from the sharp “business end” to the molded plastic handle, as well as assembly, sterilization, and packaging. The company’s headquarters are located in Franklin, Massachusetts, and it has manufacturing facilities in Massachusetts, Mississippi, Costa Rica, Europe, and Asia. Tegra Medical holds ISO 13485 and FDA registrations and is a member of SFS.
Cadence Device
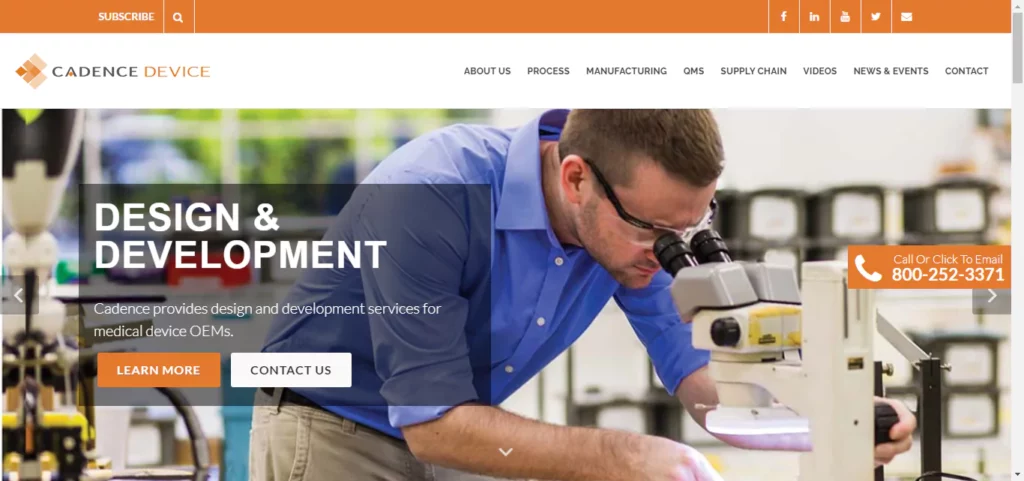
Cadence is a contract manufacturing company that specializes in serving medical and drug delivery device OEMs all over the world. They offer a wide range of product realization solutions, including contract manufacturing services, production of finished devices, complex sub-assemblies, and mission-critical components made of plastics and metals. These services are designed to improve product performance and economics. Cadence collaborates closely with customers, employees, and shareholders to deliver innovative advances in science and engineering. In addition to its expertise in custom OEM products and medical device manufacturing, Cadence also serves the automotive, defense, and industrial markets. The company markets high-performance specialty blades and precision products for the life sciences under various brand names. With a workforce of over 700 and headquarters in Staunton, VA, as well as additional facilities in several U.S. states and Costa Rica, Cadence is committed to excellence and innovation in its field.
Coghlin
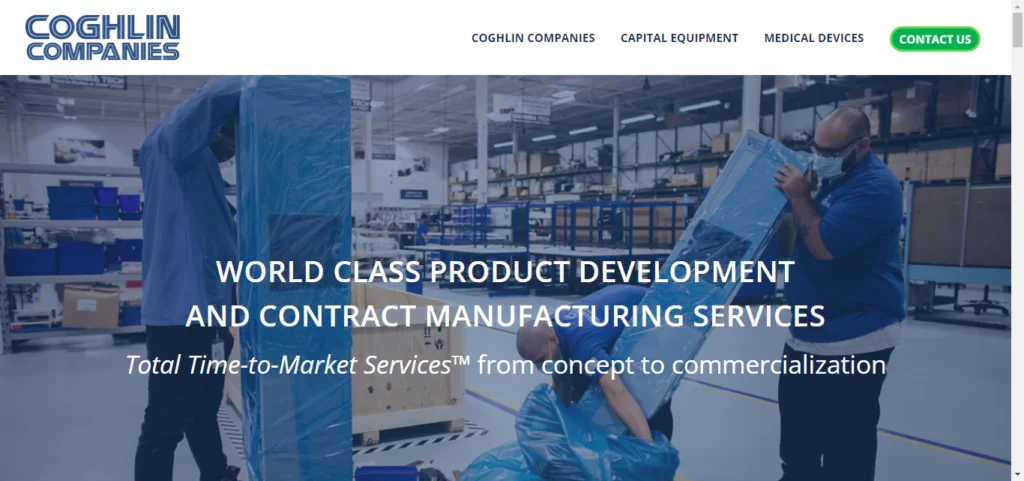
Coghlin Companies offers specialized Time to Market Services™ (TTMS) aimed at efficiently bringing industrial capital equipment and FDA-compliant medical devices to market. Through its subsidiaries, Columbia Tech and Cogmedix, Coghlin provides a comprehensive array of services including engineering, product development, manufacturing, global fulfillment, and aftermarket support under the TTMS model. Serving a broad clientele spanning industries such as medical, life sciences, robotics, industrial automation, 3D printing, advanced energy, security, military, semiconductor, LED, and pharmaceuticals, Coghlin ensures that innovators across diverse sectors successfully and efficiently launch their products into the market.
Providence Enterprise
Providence is a full-service contract manufacturer that focuses on improving the brand of its OEM customers through innovation, quality, and continuous improvement. The company is committed to the production of highly engineered finished goods and electro-mechanical assemblies. Providence is registered in Hong Kong, runs manufacturing facilities in China, and has sales and administrative offices in Hong Kong, the USA, and Europe. The company collaborates with some of the world’s largest companies and offers comprehensive contract manufacturing solutions for various industries, including Medical, Automotive, Industrial, Consumer Goods, and Electronic/Electro-Mechanical sectors.
Donatelle
Donatelle is a leading contract manufacturer that specializes in producing life-saving and enhancing medical devices. For over 50 years, they have served some of the biggest names in the medical industry, adhering to stringent regulatory standards for complex applications, including implants and disposables. Donatelle has an exemplary track record of delivering products on time, boasting a 99% on-time delivery rate. As an FDA-registered entity, Donatelle operates state-of-the-art facilities with ISO-certified cleanrooms, offering comprehensive solutions from a single process to complete manufacturing services. They have a highly experienced team across various medical markets such as cardiac, diagnostics, and orthopedics.
Janco Electronics Inc.

Janco Electronics Inc. is a veteran-owned company that specializes in providing electronics manufacturing services, particularly excelling in the medical device sector. Established in 1959, the company offers an extensive array of contract manufacturing services, focusing on assembly and high-reliability electro-mechanical systems for leading entities in the medical, military, commercial, industrial, RF, and microwave industries. Janco Electronics Inc. provides full turnkey and consignment programs customized to meet the unique needs of the medical device industry. The company’s advanced test capabilities ensure that all products meet stringent quality metrics. Janco Electronics Inc. is committed to consistently providing high-reliability and valuable electronics manufacturing services, driven by a commitment to leadership, employee development, and continuous improvement, all while upholding a strong sense of social responsibility to the community.
BMP Medical
BMP Medical is a trusted medical device manufacturer located in Sterling, Massachusetts, specializing in plastic injection molding services. As a privately held company with over 45 years of experience and veteran ownership, BMP Medical offers custom OEM injection molded medical devices with precision and quality. The company is equipped with advanced molding techniques and machinery to produce small to medium-sized parts that meet the exact specifications needed for medical devices. BMP Medical also provides a comprehensive range of services, including design consultation, engineering, R&D, tooling, mold development, computer automation, and statistical process control tailored specifically for the medical device sector. BMP Medical’s facility features a Class 8 clean room to ensure the highest cleanliness standards for medical device manufacturing, packaging, and assembly. BMP Medical values lasting relationships with its OEM medical contract manufacturing customers, some of which have been maintained for over 20 years. The company is always looking to establish new relationships within the medical device industry.
BihlerMed
BihlerMed is known for its innovative approach to designing, developing, and manufacturing medical devices. The company is committed to safety, quality, and excellence in all its endeavors. Its mission is to create advanced medical devices and accessories that contribute to improving health outcomes. The company’s motto is ‘Make Healthier Happen.’ One of BihlerMed’s notable innovations is the BihlerMed Surgical Light, which is a first-of-its-kind battery-powered LED surgical lighting line. This innovative product provides superior illumination and visualization, addressing the limitations found in traditional surgical lighting solutions within the operating room environment. BihlerMed’s contributions to the medical field enable surgeons to conduct procedures more efficiently and safely, leading to enhanced patient outcomes. The company plays a pivotal role in advancing surgical practices.
HDA Technology
HDA Technology is a company that was established in 1982, and they specialize in creating innovative medical products. They offer comprehensive design and manufacturing services for Class-I, II, and III medical devices, and they are certified by ISO13485, FDA, FDB, AS9100, and ISO9001. HDA operates from an FDA-registered facility located in Orange County, California. They cater to startups, mid-sized companies, and multinational corporations and can handle various production volumes, from small batches for clinical trials to large-scale commercial production. The company is committed to quality, and they demonstrate this through their design process, ensuring their products meet stringent standards and requirements. HDA incorporates Design for Compliance and conducts in-house pre-compliance testing, streamlining the certification process and ensuring their products are market-ready in an efficient manner. They demonstrate a blend of innovation, quality, and regulatory expertise in their work.
DeviceLab
DeviceLab is a highly experienced and reputable medical device design and development firm that has been operating in the industry for over 20 years. They have received ISO 13485 certification and have completed over 100 significant projects and 400 smaller ones, giving them a deep understanding of FDA requirements and ISO standards. Their portfolio includes wearable medical devices, LED and laser products, patient monitors, and advanced imaging systems, catering to a variety of needs in home, Point of Care (POC), or hospital settings. DeviceLab has been recognized with several awards and works with a diverse range of clients, from startups to Fortune 100 corporations, to deliver market-driven designs quickly and cost-effectively.
Emerging Trends in Medical Device Contract Manufacturing
The world of medical device contract manufacturing is changing fast, thanks to some exciting new trends that are changing things rapidly. Here’s what’s happening:
- New Technologies: Innovations like 3D printing, artificial intelligence (AI), and the Internet of Things (IoT) are changing how medical devices are made. They’re making it possible to create personalized and more efficient healthcare solutions.
- Sustainability: There’s a growing focus on being eco-friendly in manufacturing. This means using materials and processes that are better for the environment. It’s part of a bigger trend towards being more responsible for our planet.
- Wearable Devices: More and more people are using wearable medical devices and products for healthcare at home. This includes features such as fitness trackers and devices that monitor health conditions. It’s all about taking a proactive approach to healthcare and keeping an eye on your health outside of the doctor’s office.
- Changing Regulations: Rules and regulations for making medical devices are always changing. Governments around the world are making them stricter to ensure safety, quality, and traceability.
All these trends show that the medical device contract manufacturing industry is going through some big changes. It’s a time of innovation, flexibility, and making sure everyone follows the rules.
The Future of Medical Device Contract Manufacturing
The future of medical device contract manufacturing looks promising, thanks to rapid technological progress and increasing healthcare needs. Analysts predict that the global medical device market will grow by around 5% each year, indicating significant growth in development and manufacturing. To prepare for this growth, companies are investing in advanced technologies like 3D printing and AI, which will make manufacturing more efficient and allow for more customized products. The focus on sustainability and eco-friendly practices is also driving innovation in materials and processes, which will further fuel market expansion. Strategic partnerships and collaborations, especially those connecting technology with healthcare, will be crucial for navigating regulations and creating new solutions. These partnerships will create an environment where medical device contract manufacturing innovation can thrive, and companies can meet market demands with precision and flexibility.
Conclusion
In conclusion, the medical device contract manufacturing sector stands at the cusp of transformation, driven by technological advancements, shifting market demands, and sustainability concerns. Adapting to global regulations and healthcare shifts, embracing new technologies, forming strategic partnerships, and prioritizing eco-friendly practices are crucial for shaping its future.
Medical brands increasingly rely on contract manufacturers for innovation. Companies that invest in innovation and collaboration will lead, exceeding expectations with medical devices that redefine possibilities.
The future of medical device contract manufacturing promises personalized, efficient, and accessible healthcare solutions, playing a vital role in advancing global healthcare and building a healthier world. Through innovation and sustainability, these manufacturers will continue to lead transformative advancements in the industry.